"The field of research and development for the electro-chemist will be practically limitless, and will extend beyond what the world is now considering—the 'conservation of natural resources'—for they will create valuable products from wastes not now dignified by the title of resources."
In 1910, upon receiving the Society of Chemical Industry’s prestigious Perkin Medal for “innovation in applied chemistry resulting in outstanding commercial development,” Dr. Edward Goodrich Acheson described the state of industrial development. As he pointed out, our world evolved from ages dominated with single technologies based in stone, bronze, and iron to a time when science developed a myriad of materials which supported its continuing technological expansion. Creating industrial materials had been largely limited to relatively crude methods fashioned in earlier times. Industrial metal alloys such as steel from the Iberian Peninsula (4th century BCE) and brass from Ancient Greece (1st century BCE) had been around for centuries; synthetic silicon carbide, however, was only brought to realization in 1893 by Dr. Acheson. In particular, expanding from compartmentalized, single technologies into multidisciplinary fields defined the industrial expansion of the late 1800s and into the new century. Over the succeeding century, Acheson’s “bright specks” and other electrochemical production techniques transformed industries.
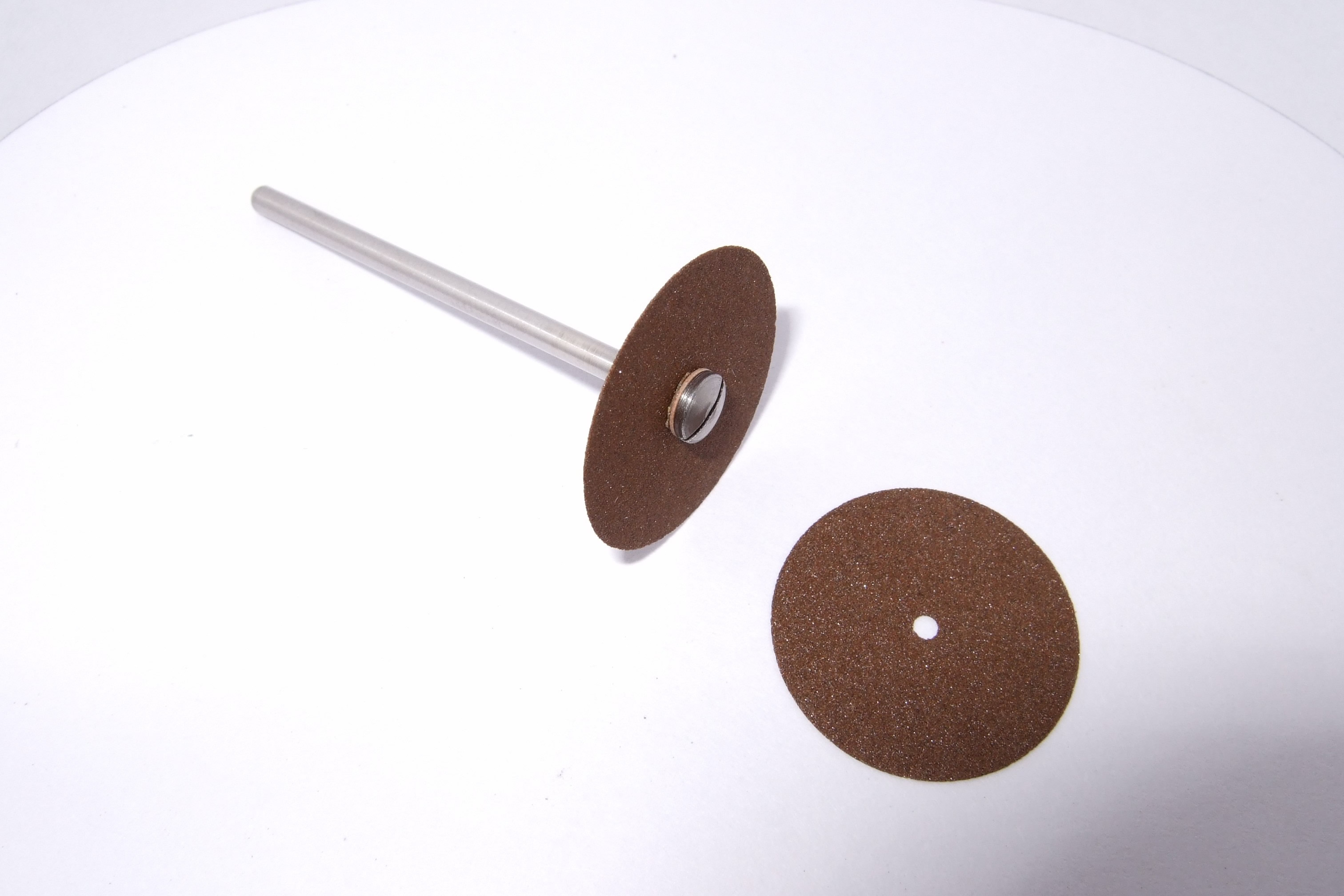
While struggling to develop cubic zirconium (a substitute for the much more rare and expensive diamond), Pittsburgh native, industrialist, and chemist Edward Goodrich Acheson accidently discovered the process for synthesizing one of the hardest compounds now known to humanity—silicon carbide. Silicon carbide (SiC) was among the first materials industrialized by electro-chemical production which has since been adapted as a standard process in the materials industry. Acheson introduced his research to the public through heating elements and abrasives, but the technology has expanded into an entire industry focused on serving many fields.
Due to its high temperature resistance, silicon carbide composites are used in high performance carbon-carbon composite automobile brakes, high temperature gas turbines, and various furnace applications. In pure cubic form, silicon carbide has become a standard material in high performance semiconductor applications. First used in light-emitting diodes in the mid 20th century, advanced composites are now employed throughout electronics in Schottky diodes, MOSFETs, and high temperature thyristors. Due to silicon carbide’s other physical properties such as low thermal expansion coefficient (minimal size change over a wide range of temperatures), high rigidity, and thermal conductivity (ability to transmit heat), silicon carbide has become a standard material for space-based mirror systems. Silicon carbide was particularly important during the hey-day of Pennsylvania’s steel industry since manufacturers would add it to the steel to adjust the tap temperature and the carbon content of the steel.

In his autobiography, Acheson described his childhood as filled with “strictly boyish pleasures,” but after only three years of formal education, at the age of 17, he was forced into the working world in the wake of the financial crisis of 1873. Acheson was born on May 9, 1856, into the Washington, Pennsylvania home of grocers William and Sarah Diana Acheson. Five years later, the family moved to Monticello (about 47 miles north of Pittsburgh in Butler County) where William was made manager of “Monticello Irons,” a furnace purchased by Edward’s uncle and father, among several others. In 1869, Edward attended boarding school, but by the next year, he transferred to the academy in Bellefonte, Centre County. While attending the academy, Edward realized his “fondness for mathematics,” particularly geometry, establishing his interest in engineering. In the fall semester of 1872, predicting the economic downturn, Edward’s father called him home, ending his formal education. By 1874, the depression sent his father’s estate, held by an iron company, into bankruptcy.
“I think it was in 1873, when 17 years old, I made my first practical acquaintance with Electricity,” Acheson recollected in his autobiography. “I bought a number of cheap yellow metal watches. Fitting up galvanic batteries, I secured a number of my mother’s silver forks and with them as anodes, silver plated the watch cases, and sold them at an increased price. When my mother’s effects were divided among her children, my ‘anodes’ were properly deducted from my allotment.” Throughout his 20s, Acheson developed a particular interest in electrical science and left his lucrative job with United Pipe Lines to seek employment in the electrical industry with Edward Weston, but was not offered a position.
In desperation for a job, Acheson went to the Edison Company to speak with Thomas Edison. Acheson recounted his introduction: “‘What do you wish?;’ I replied ‘Work.’ He replied, with perhaps impatience, ‘Go out to the machine shop and see Kruesi.’” After working on a variety of projects in Edison’s company, Edison promoted Acheson to the original experimental department. “I was now in my glory,” he said. Acheson became a master in the lamp business and was sent to Europe for several years to share his knowledge with Edison’s subsidiary companies overseas. Upon returning to the United States, Edison reassigned Acheson into his experimental research laboratory, now located in New York City, but once Acheson’s research interests differed from Edison’s, he left the company.
After failing to produce synthetic rubber, Mr. John S. Huyler, financier of the research, redirected Acheson’s focus to the development of an abrasive. Drawing upon his previous research, Acheson began his investigation by introducing carbon to clay under electric heat. He attached a dynamo, a DC electric generator, to an iron bowl filled with clay and powdered coke and inserted the opposite end of an arc light carbon into the mixture. After a strong current passed through the resistive mixture sufficiently to melt the material, Acheson was disappointed to find that the cooled chunk was not representative of his expectations, but noticed a “few bright specks at the end of the arc carbon.” Acheson drew one of the crystalline samples across a pane of glass, and found surprisingly similar results to diamond. Having yet to conduct chemical composition analysis on the unknown abrasive material, Acheson named it “Carborundum” on the false assumption that its composition was of carbon and corundum. A few months later he would learn its true formula—silicon and carbon, SiC.
Having sent his material to several emery wheel manufacturers and hearing that it was impossible to produce wheels from carborundum, Acheson founded and incorporated the Carborundum Company on September 21, 1891 with $150,000 to conduct his own research. The company’s small cutting wheels found their first large scale application in Westinghouse Electric’s ground two piece lamps, affording the Carborundum Company enough capital to purchase its own dynamo. Following, in 1893, Acheson expanded his market into dentistry tools.
Within the decade, Acheson sold his international patents to wealthy investors throughout Europe. Upon his return to the United States, Acheson learned of a new electric production plant on Niagara Falls and sought to move the Carborundum Company from its factory with a dynamo that produced 134 horsepower to a 1000hp facility in New York. When the conservative board rejected the proposal and resigned from its duties, the company’s future was left to Acheson who compiled a new board supporting the allocation of $75,000 worth of bonds to supplement Acheson’s own personal investment from his European patent sales. Following months of financial shortages, in the fall of 1895, the Niagara Falls facilities were completed.
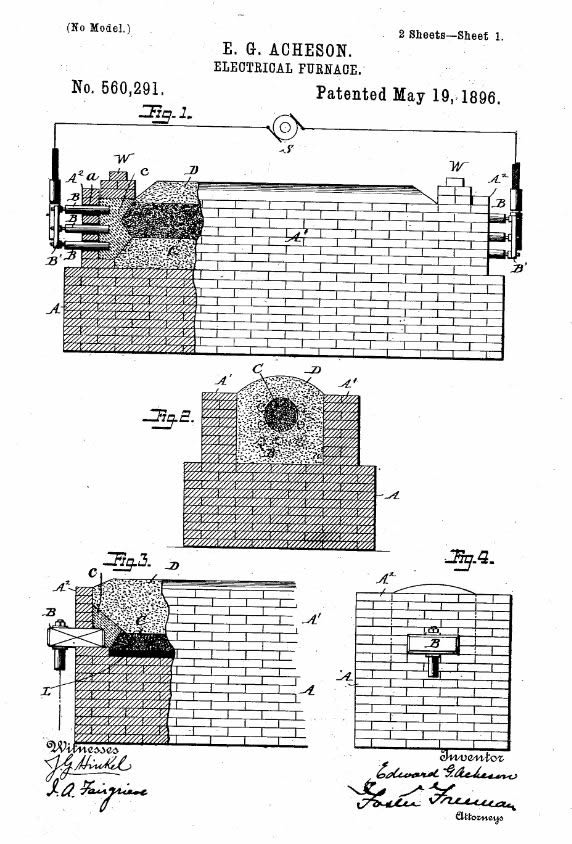
Acheson refined his initial experiment and patented the Electrical Furnace on May 19, 1896 (No. 560,291). The electric furnace improved efficiency and economy, promoting the Carborundum Company into the world’s premiere (and only) manufacturer of industrial quantities of silicon carbide. In this new process a large current passes through electrodes attached to a graphite core within a mixture of sand and salt. The resistance from the materials generates heat, leading to the formation of a hollow silicon carbide cylinder around the graphite core. The heat provides sufficient energy for silicon oxide (SiO2) and carbon to react and yield silicon carbide. By increasing the size of the furnace and, thus, its electrical demands, the system’s output can be scaled to meet production requirements and electrical limitations.
In this electro-chemical process, the crystal structure of the silicon carbide molecules can be coerced into different compositions depending on the specifications required: temperature, reaction duration, chemical composition, and current strength. While Acheson believed the transformation occurred due to a condensation phase reaction between SiO2 and C, researchers have since understood that SiC is the product of a series of reactions. With this reaction sequence in mind, scientists can prepare mixtures of precise chemical composition through particular manipulation of the system’s parameters, thus improving the efficiency and quality of the silicon carbide production process.
As the Carborundum Company expanded through the generous support of a few investors to include wheel and paper departments (think grinding wheels and sandpaper), Acheson and his administrative team were completely removed from the company by 1901. Nonetheless, Acheson was the founder of a sound company and father of a new industrial field. After being replaced by Mr. F. W. Haskell on July 1st, Acheson described his contribution: “I had created an entirely new industry, worked out and patented the many details of manufacture, created a stock to supply demands from the trade, proved the value of Carborundum as an abrasive and established a demand for the same, and all this while the country was passing through a great financial depression.” By 1910, the Carborundum Company was running on 10,000hp of electricity and was producing ten million pounds of silicon carbide per year. By 1930, the Carborundum Company was supplying the world with enough silicon carbide to influence the entire metallurgical, stone, abrasive paper, leather, jewelry, rice, and high temperature electrical heating element industries. And this was only the beginning.
While most materials take many years, if not generations, to harness the public’s support, Dr. Acheson’s synthetic silicon carbide received praise from both professional institutions and the public almost instantly. Just a few of the notable honors bestowed upon him for his research on silicon carbide include the John Scott Medal from the City of Philadelphia and the Franklin Institute (1894), the Gold Medal from the Louisiana Purchase Exposition (1904), the Perkin Medal from the American section of the Society of Chemical Industry (1910), and the aptly-named Acheson Medal by the American Electrochemical Society (1929).
In 1930, the Smithsonian Institution honored Acheson with his own exhibit showcasing his influence in electro-chemistry. Acheson would die the next year on July 6, 1931. Even laymen recognized Acheson’s contribution. One man wrote to the editor of the New York Times citing the importance of silicon carbide in promoting the development of other revolutionary technologies of the time: “Without this important abrasive-a low-cost product next to the diamond in hardness—mass production of the automobile, airplane and other mechanical devices…would have been impossible.”
The carborundum process as sparked by Edward Goodrich Acheson not only moved silicon carbide into commercial production but facilitated the entire electrochemical industry sparking the industrial expansion of the early twentieth century. Acheson’s work in the development of furnace technology has been adapted for use in similar technologies for the production of many electro-chemically produced materials. Silicon carbide’s influence on the world has also evolved to match Acheson’s own words: “The field of research and development for the electro-chemist will be practically limitless, and will extend beyond what the world is now considering.” Expanding from its initial applications, silicon carbide has infiltrated the fields of electronics, aeronautics, military armor, sensitive space based instrumentation, and nuclear fuel elements. As research continues over a century later, the applications of Acheson’s “bright specks” will continue to expand into yet unknown industries.
In 1926, the United States Patent Office displayed an exhibit of the “most important patents that have influenced the world’s progress.” Among the Wright Brothers’ airplane, Morse’s telegraph, and Bell’s telephone, stood Acheson’s silicon carbide. “This artificial material, harder than any other substance except one or two precious and costly materials, has a noteworthy influence as an abrasive for its cutting qualities.”
The Center wishes to thank Jake Smail, ME for his assistance with this article.
Sources:
- Acheson, Edward G. A Pathfinder: Discovery, Invention and Industry. New York: Scrap Book, 1910.
- Acheson, Edward G. “Carborundum: Its History, Manufacture, and Uses.” The Journal of the Franklin Institute Devoted to Science and the Mechanic Arts 136 (1893): 194+.
- Adler, J., G. Standke, M. Jahn, and F. Marschallek. “Cellular Ceramics Made of Silicon Carbide Ceramics for Burner Technology.” Advances in Bioceramics and Porous Ceramics: Ceramic Engineering and Science Proceedings 29.7 (2009): 271-86.
- Carborundum Abrasives Products. 15 Oct. 2010 <http://www.carborundumabrasives.com>.
- Dumych, Daniel M. “Edward Goodrich Acheson.” American National Biography Online. Oxford UP, Feb. 2000. 10 Oct. 2010 <http://www.anb.org/articles/13/13-00007.html>.
- “Dr. Acheson Dies; Eminent Scientist.” New York Times 7 July 1931: 19.
- Electrical Furnace. Edward Goodrich Acheson, assignee. Patent 560291. 19 May 1896. 23 May 2011 <http://www.uspto.gov>.
- “Gives $25,000 to Science.” New York Times 23 Sept. 1928: 20.
- Griffin, Timothy E. Pulsed Capacitance Measurement of Silicon Carbide Schottky Diode and SiC Metal Oxide Semiconductor. Tech. no. ARL-TR-3993. Adelphi: Army Research Laboratory, 2006.
- Halbig, Michael C., and Mrityunjay Singh. “Development and Characterization of the Bonding and Integration Technologies Needed for Fabricating Silicon Carbide Based Injector Components.” Advanced Processing and Manufacturing Technologies for Structural and Multifunctional Materials II: Ceramic Engineering and Science Proceedings 29.9 (2009): 1-14.
- International Conference on Silicon Carbide, (1968: Pennsylvania State University). Silicon Carbide-1968: Proceedings. New York: Pergamon, 1969.
- LaSalvia, Jerry C., Brian Leavy, Herbert T. Miller, Joshua R. Houskamp, and Ryan C. McCuiston. “Recent Results on the Fundamental Performance of a Hot-Pressed Silicon Carbide Impacted by Sub-Scale Long-Rod Penetrations.” Advances in Ceramic Armor IV: Ceramic Engineering and Science Proceedings 29.6 (2009): 89-97.
- Production of Artificial Crystalline Carbonaceous Materials. Edward Goodrich Acheson, assignee. Patent 492767. 28 Feb. 1893. 23 May 2011 <http://www.uspto.gov>.
- Research and Development Division of the Carborundum Company. Bibliography on Silicon Carbide. Niagara Falls, NY: Carborundum Company, 1959.
- Shur, Michael, Sergey L. Rumyantsev, and M. E. Levinshtein. SiC Materials and Devices. Hackensack: NJ: World Scientific, 2006.
- Smail, Jake. Personal Communication with Alan Jalowitz. 25 May 2011.
- Smithsonian Institution, ed. The Edward Goodrich Acheson Exhibit. Port Huron, MI: Acheson Oildag, 1930.
- Szymanowitz, R. “Silicon Carbide.” New York Times 2 Dec. 1932: 20.
- “The Manufacture of Carborundum- a New Industry.” Scientific American 7 Apr. 1894: 215.
- Weimer, Alan Wesley. Carbide, Nitride, and Boride Materials Synthesis and Processing. London: Chapman & Hall, 1997.
- Wendel, William H. The Scratch Heard ’round the World: The Story of the Carborundum Company. New York: Newcomen Society in North America, 1965.